Insurance Listing

Evidence of cost-effectiveness and clinical utility is required.
The structure of Japan’s health insurance system is as follows:
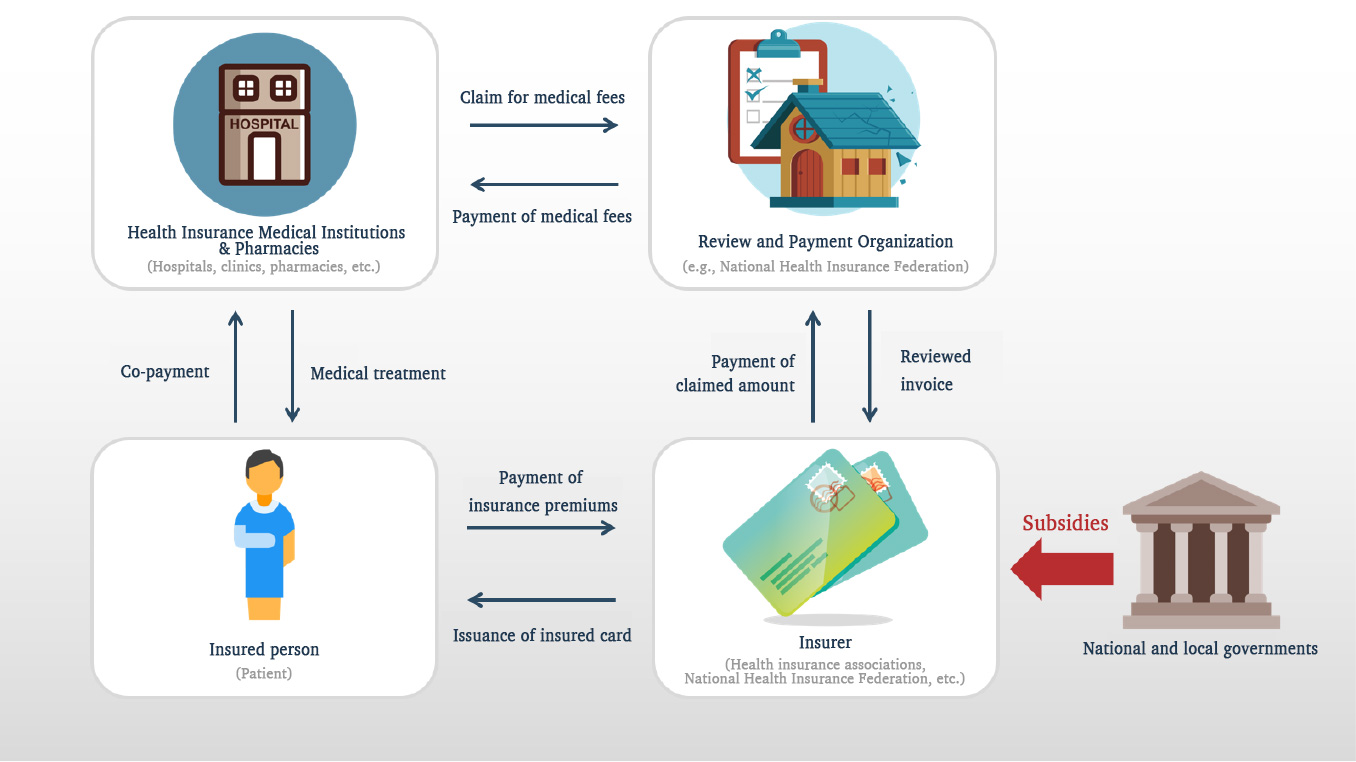
Picture 1: Structure of Japan’s Health Insurance System (Created by JOMDD)
Under Japan’s health insurance system, the amount that medical institutions can charge for insured medical services is fixed. This amount is known as the “medical fee.”
The medical fee is set by the Minister of Health, Labour and Welfare, based on the recommendations of the Central Social Insurance Medical Council (Chuikyo), which serves as an advisory body to the Minister. In general, these fees are reviewed and revised every two years.
The term “medical fee” mainly refers to the “technical fee”—the payment for the professional act of providing medical services. In addition, the prices of the materials and devices used in medical procedures are determined separately. These are categorized as “specific health insurance medical materials,” which include certain medical devices and disposable supplies.
The classification of insurance in Japan is as follows:
A1 (Comprehensive)
Evaluated comprehensively under existing medical fee items (e.g., sutures, venipuncture needles)
A2 (Specific Comprehensive)
Evaluated comprehensively under specific existing medical fee items (e.g., ultrasound equipment and ultrasound examinations)
A3 (Existing Technology with Modifications)
Evaluated under existing medical fee items for the technology using the product
B1 (Existing Functional Part)
Evaluated according to existing functional categories, separately from technical fees (e.g., coronary stents, pacemakers)
B2 (Existing Functional Part with Modifications)
Evaluated according to existing functional categories, separately from technical fees (including changes to the definitions of functional categories)
B3 (Time-Limited Improvement Addition)
Evaluated by adding a time-limited improvement fee to existing functional categories
C1 (New Function)
A new functional category is required,but the technology using it has already been evaluated (e.g., artificially processed joint implants)
C2 (New Function / New Technology)
The technology using the product has not been previously evaluated(e.g., leadless pacemaker)
Note: B3, C1, and C2 are evaluation categories that require approval by the Central Social Insurance Medical Council (Chuikyo).
Source: Central Social Insurance Medical Council, Subcommittee on Health Insurance Medical Materials, “Review of the Health Insurance Medical Materials System (Part 1)” (September 20, 2023)






